AngioJet™
Thrombectomy System
Objectives
- Determine efficacy of thrombus removal from baseline to final angiogram/venogram
- Evaluate clinical outcomes of treated patients at defined intervals of 3, 6 & 12 months
- Characterize clinical events
- Characterize treatment options used with the AngioJet System
- Estimate rate of AngioJet Thrombectomy-related adverse events
Peripheral Venous Thrombus
Post Thrombotic Syndrome (PTS) is a chronic, debilitating complication of DVT occurring in 20-50% of patients following a proximal DVT.3
The AngioJet System provides the power and flexibility to remove thrombus and restore flow in even the most challenging DVT cases.
Recent PEARL Registry data showed:1
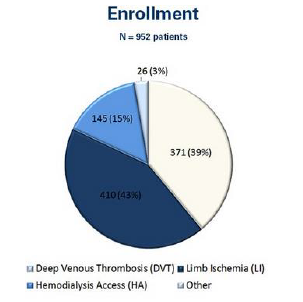
- AngioJet Thrombectomy removed 96% of thrombus in lower extremity veins with 84% freedom of re-thrombosis after 1 year 86% of cases utilized Power Pulse and/or Rapid Lysis approach (N=371 patients)
- Less lytic and shorter procedure times using either Power Pulse or Power Pulse plus CDT than with CDT alone with AngioJet
- 87% of AngioJet venous cases were completed in 2 or less sessions, and 75% of cases were completed in less than 24 hours
AngioJet Percutaneous Mechanical Thrombectomy for DVT can result in less treatment time and cost efficiencies compared to traditional CDT.2
Peripheral Arterial Thrombus
Acute Limb Ischemia (ALI) remains a life-threatening condition with 9% and 15% in-hospital and 30-day mortality rates, respectively; and 15% and 25% amputation rates at discharge and 30 days.4,5
AngioJet Thrombectomy removes clot burden from arterial vessels as small as 1.5mm–restoring flow, and resolving symptoms while exposing the culprit lesion, facilitating treatment.
Recent PEARL Registry data showed:1
- Immediate improvement in 93% of arterial vessels treated
- 89% (185/207) limb salvage rate for patients presenting with threatened limbs at baseline (Rutherford classifications of IIa, IIb and III).
AV Access Conduits
Thrombus narrowing or restricting flow within AV access fistulas and grafts can prevent a patient from undergoing life supportive dialysis treatment.
Used for thrombectomy of both synthetic grafts and natural fistulae, the AngioJet System utilizes powerful Cross-Stream technology to remove thrombotic materials from the dialysis access conduit with minimal vessel wall trauma, potentially decreasing the risk for future thrombotic events.
Catheters with AV access indication include: AVX, Solent Proxi and Solent Omni.
Recent PEARL Registry data showed:1
- Patency and functionality rate of 78% at 3 months compared to KDOQI guidelines target of 40%
- 97% of treated AV vessels showed imporved angiographic results (N=186 vessels)
- Patients maintained graft / fistula survival of 76% after 1 year