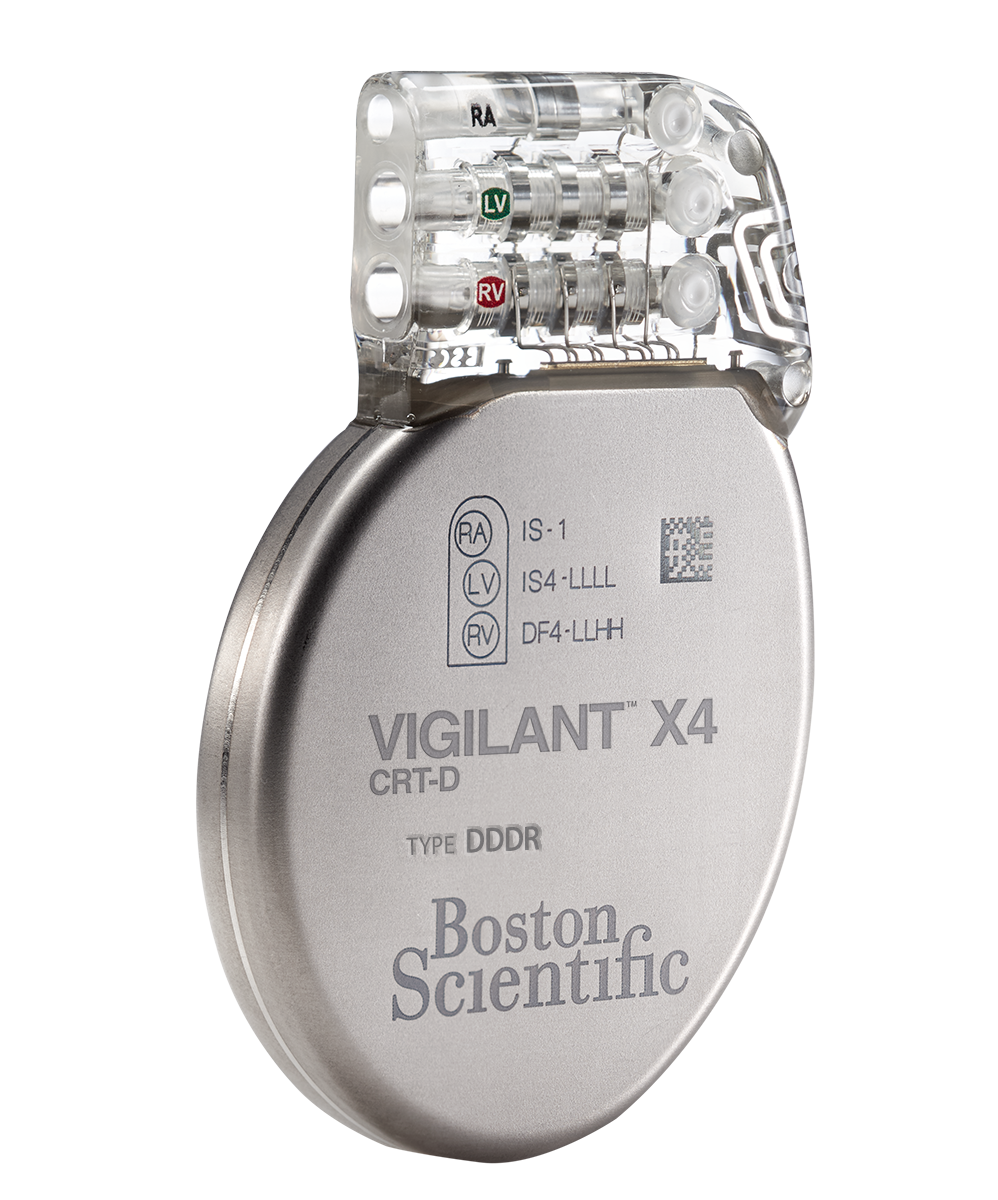
VIGILANT™ X4 CRT-D and
VIGILANT™ CRT-D
Cardiac Resynchronization Therapy Defibrillator
SmartCRT™ is Boston Scientific’s approach to personalise CRT therapy by providing physicians with smart solutions to optimise where, when, and how to pace. HeartLogic™, the first and only heart failure diagnostic validated to have high sensitivity, the ability to provide weeks of advanced notice and low alert burden for detecting early signs of worsening heart failure.* EnduraLife™ Battery Technology provides more power to use more of the device, featuring up to 13.3 years projected longevity with MultiSite Pacing turned on.** ImageReady™ MR Conditional Systems allow patients to safely undergo Full Body MRI scans up to 1.5T and 3T.***
Explore
Product Details
VIGILANT™ X4 CRT-D and VIGILANT™ CRT-D
Models G224 and G247

- VIGILANT X4 CRT-D offers SmartCRT™ technology, enabling physicians to offer a personalised care approach to all patients, including a wide range of options of where, when and how to pace
- EnduraLife™ battery technology can offer up to 14.7 years battery life1 and 13.3 years with MultiSite Pacing switched on1.
- The X4 CRT-D and ACUITY™ X4 lead portfolio features electrodes on a 3D spiral designed to allow pacing from a basal location without sacrificing fixation or thresholds; 17 pacing vector options to manage around PNS and high thresholds; and multiple lead shapes with a 2.6F (0.86mm) tapered lead tip to improve predictability of accessing target vessels.
- MultiSite Pacing allows 216 vector combinations to easily tailor device therapy, supported by automatic recommendations with the click of a button, for vectors and offsets in < 5 seconds.
- This small (32.5 cm3) and thin (9.9 mm) high-energy device is designed to enhance patient comfort.2
- The PaceSafe™ RV, RA, and LV auto threshold feature is designed to maintain adequate output safety margins.
- Heart Failure Sensor Suite with a set of tools such as: Sleep Incline, LATITUDE™ NXT Remote Patient Management with weight scale and blood pressure sensors and Respiratory Rate Trend, to provide an advanced solution for patient comorbidities and HF monitoring.
- ImageReady™ MR-Conditional systems allow patients to receive full body MR-Conditional scans at 1.5T or 3T without exclusion zone, scan duration, or patient height restrictions**
- AcuShock™ Technology including Onset/Stability™, RhythmID™, Dynamic Noise Algorithm (DNA) for sensing, Automatic Gain Control (AGC) with programmable sensing floor, Narrow Band Pass Filter
- EasyView™ header and color coded lead ports designed to make the implant experience more efficient.
- SafetyCore™ technology is intended to provide lifesaving shock therapy and basic pacing functionality in the event of an unrecoverable fault.
Mechanical Specifications
| Model | Type | Size (cm) (W x H x D) | Mass (g) | Volume (cc) | Connector Type (RA RV LV) | MR Conditional |
|---|---|---|---|---|---|---|
| G247 | X4 CRT-D | 5.37 x 8.18 x 0.99 | 73.8 | 32.5 | RA:IS-1;RV:DF4;LV:IS4 | Yes |
| G224 | CRT-D | 5.37 x 8.18 x 0.99 | 73.6 | 32.5 | RA:IS-1;RV:DF4;LV:IS-1 | Yes |
Pulse Generator Longevity (All Modelsa,b,c,d,e,f)
EnduraLife™ Battery Technology provides clinically-proven, industry-leading projected longevity.*** The following tables represent sample pulse generator life expectancy estimation (implant to explant) with EnduraLife™ battery as provided in product labeling. For specific programmable parameter ranges, refer to product labeling at www.bostonscientific-elabeling.com, or contact Boston Scientific technical services or your local representative.
MultiSite Pacing Off
| Projected longevity | Ventricular Chambers | RA/RV | LV | LVbᵉ | 500Ω with Latitude™ᶜ | 700Ω with Latitude™ᶜ | 700Ω no Latitude™, MV/RS, or HFSSᵈ |
|---|---|---|---|---|---|---|---|
| Typical programmed setting | BiV | 2.5 V | 3.0 V | Off | 9.7 | 10.5 | 11.3 |
| Maximum labelled longevity | LV-Only | 2.0 V / Off | 2.0 V | Off | 12.9 | 13.2 | 14.7 |
MultiSite Pacing On
| Projected longevity | Ventricular Chambers | RA/RV | LV | LVbᵉ | 500Ω with Latitude™ᶜ | 700Ω with Latitude™ᶜ | 700Ω no Latitude™, MV/RS, or HFSSᵈ |
|---|---|---|---|---|---|---|---|
| Typical programmed setting | BiV MSP | 2.5 V | 3.0 V | 3.0 V | 8.2 | 9.1 | 9.7 |
| Maximum labelled longevity | LV-Only MSP | 2.0 V / Off | 2.0 V | 2.0 V | 11.5 | 12.1 | 13.3 |
Additional Longevity Information
- Warranty information available at www.bostonscientific.com/warranty.
- Devices use Li/MnO2 chemistry.
- The Usable Battery Capacity is 1.9 Amp-hours (typical implant to battery capacity depleted).
- Shelf life is 2 years (before use by date).