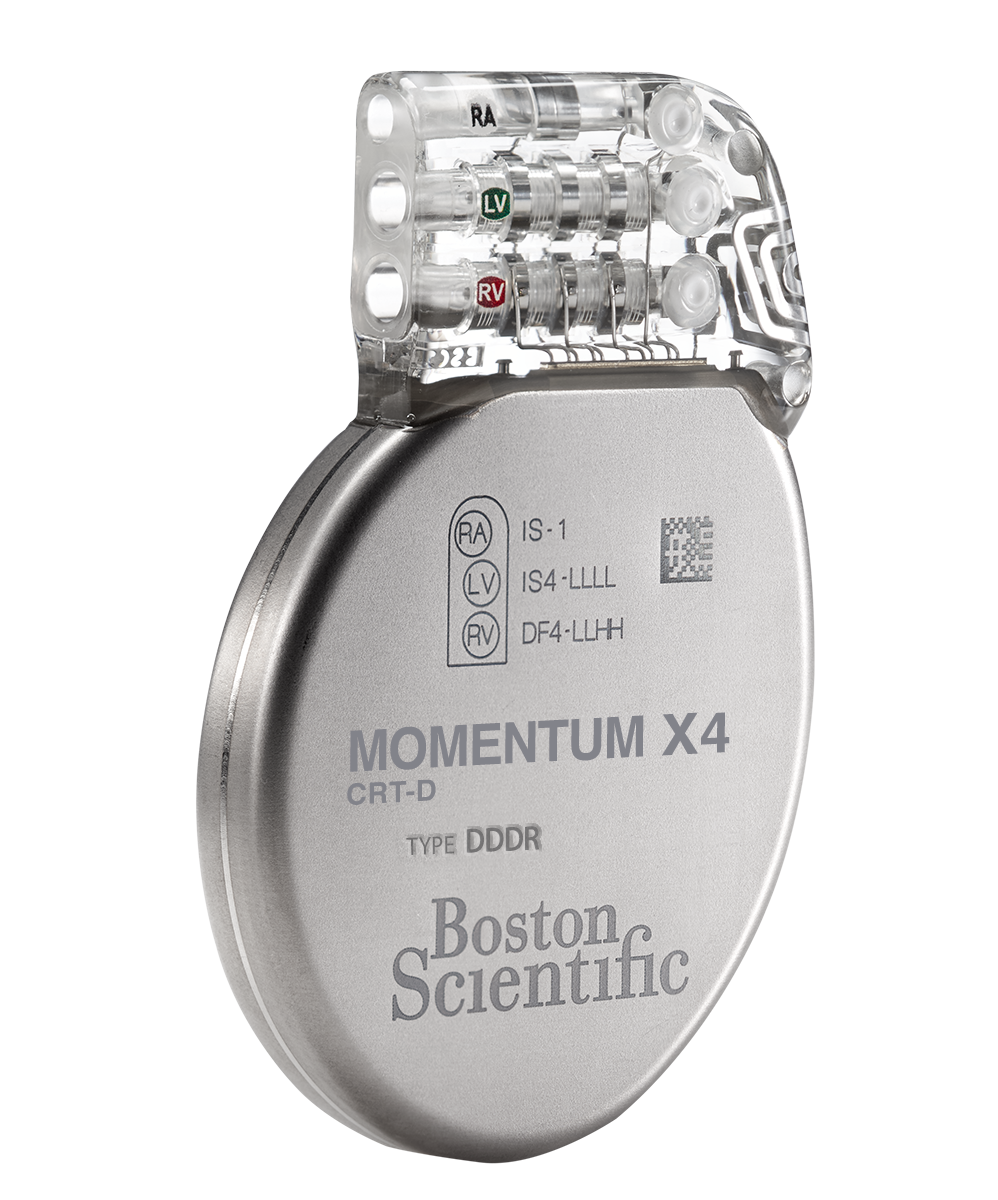
MOMENTUM™ X4 CRT-D and
MOMENTUM™ CRT-D
Cardiac Resynchronization Therapy Defibrillator
SmartCRT™ is Boston Scientific’s approach to personalise CRT therapy by providing physicians with smart solutions to optimise where, when, and how to pace. EnduraLife™ Battery Technology provides more power to use more of the device, featuring up to 13.3 years projected longevity with MultiSite Pacing turned on.*
ImageReady MR conditional Systems allows patients to receive full body MR-Conditional scans at 1.5T without exclusion zone, scan duration, or patient height restrictions.**
Explore
Product Details
MOMENTUM™ X4 CRT-D and MOMENTUM™ CRT-D
Models G124, G125, G126 and G138

- MOMENTUM X4 CRT-D offers SmartCRT™ technology, enabling physicians to offer a personalised care approach to all patients, including a wide range of options of where, when and how to pace.
- EnduraLife™ battery technology can offer up to 14.7 years battery life1 and 13.3 years with MultiSite Pacing switched on1.
- ImageReady™ with Full-body MR scans safely in 1.5 Tesla environments**
- The X4 CRT-D and ACUITY™ X4 lead portfolio features electrodes on a 3D spiral designed to allow pacing from a basal location without sacrificing fixation or thresholds; 17 pacing vector options to manage around PNS and high thresholds; and multiple lead shapes with a 2.6F (0.86mm) tapered lead tip to improve predictability of accessing target vessels.
- MultiSite Pacing allows 216 vector combinations to easily tailor device therapy, supported by automatic recommendations with the click of a button, for vectors and offsets in < 5 seconds.
- Atrial Arrhythmia Report provides a comprehensive view of your patient’s AT/AF status and information for assessing AF treatment efficacy.
- RightRate™ features Boston Scientific’s MV Sensor—the only sensor clinically proven to restore chronotropic competence.2
- This small (32.5 cm3) and thin(9.9 mm) high-energy device is designed to enhance patient comfort.3
- The PaceSafe™ RV, RA, and LV auto threshold feature is designed to maintain adequate output safety margins.
- Offers AcuShock™ Advanced Technology, multiple programmable options to reduce inappropriate and unnecessary shocks, including a choice of rhythm discriminators, RhythmID™ with RhythmMatch™ (customization of Rhythm ID algorithm), antitachycardia pacing (ATP) therapy in all rates zones, and advanced sensing and filtering.
- Wireless ECG saves time and simplifies follow-up.
- EasyView™ header and color coded lead ports designed to make the implant experience more efficient.
- SafetyCore™ technology is intended to provide lifesaving shock therapy and basic pacing functionality in the event of an unrecoverable fault.
Mechanical Specifications
| Model | Type | Size (cm) (W x H x D) | Mass (g) | Volume (cc) | Connector Type (RA RV LV) | MR Conditional |
|---|---|---|---|---|---|---|
| G138 | X4 CRT-D | 5.37 x 8.08 x 0.99 | 73.4 | 32.0 | RA:IS-1;RV:IS-1/DF-1;LV:IS4 | Yes |
| G124 | CRT-D | 5.37 x 8.18 x 0.99 | 73.6 | 32.5 | RA:IS-1;RV:DF4;LV:IS-1 | Yes |
| G125 | CRT-D | 5.37 x 8.08 x 0.99 | 72.8 | 32.0 | RA:IS-1;RV:IS-1/DF-1;LV:IS-1 | Yes |
| G126 | CRT-D | 5.37 x 8.18 x 0.99 | 72.9 | 32.0 | RA:IS-1;RV:IS-1/DF-1;LV:LV-1 | No |
Pulse Generator Longevity (All Modelsa,b,c,d,e,f)
EnduraLife™ Battery Technology provides clinically-proven, industry-leading projected longevity.*** The following tables represent sample pulse generator life expectancy estimation (implant to explant) with EnduraLife™ battery as provided in product labeling. For specific programmable parameter ranges, refer to product labeling at www.bostonscientific-elabeling.com, or contact Boston Scientific technical services or your local representative.
MultiSite Pacing Off
| Projected longevity | Ventricular Chambers | RA/RV | LV | LVbᵉ | 500Ω with Latitude™ᶜ | 700Ω with Latitude™ᶜ | 700Ω no Latitude™, MV/RS, or HFSSᵈ |
|---|---|---|---|---|---|---|---|
| Typical programmed setting | BiV | 2.5 V | 3.0 V | Off | 9.7 | 10.5 | 11.3 |
| Maximum labelled longevity | LV-Only | 2.0 V / Off | 2.0 V | Off | 12.9 | 13.2 | 14.7 |
MultiSite Pacing On
| Projected longevity | Ventricular Chambers | RA/RV | LV | LVbᵉ | 500Ω with Latitude™ᶜ | 700Ω with Latitude™ᶜ | 700Ω no Latitude™, MV/RS, or HFSSᵈ |
|---|---|---|---|---|---|---|---|
| Typical programmed setting | BiV MSP | 2.5 V | 3.0 V | 3.0 V | 8.2 | 9.1 | 9.7 |
| Maximum labelled longevity | LV-Only MSP | 2.0 V / Off | 2.0 V | 2.0 V | 11.5 | 12.1 | 13.3 |
Additional Longevity Information
- Warranty information available at www.bostonscientific.com/warranty.
- Devices use Li/MnO2 chemistry.
- The Usable Battery Capacity is 1.9 Amp-hours (typical implant to battery capacity depleted).
- Shelf life is 2 years (before use by date).