Preclinical and Clinical Evidence for Optimal Healing and Safety with SYNERGY
SYNERGY™ is the only FDA-approved, everolimus-eluting platinum chromium stent with an abluminal bioabsorbable PLGA polymer coating designed to optimize healing.
SYNERGY demonstrates superior healing responses compared to DES in atherosclerotic rabbit iliac artery model
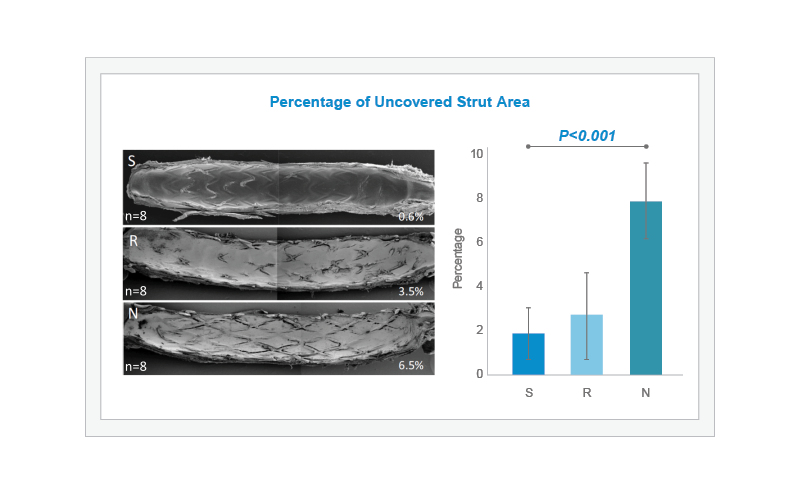
In an atherosclerotic rabbit iliac artery model, 24 rabbits were fed an atherogenic diet and their iliac arteries underwent endothelial denudation using a balloon catheter. Bioabsorbable-polymer coated DES (BP-DES) SYNERGY (S, n=8), permanent polymer-DES Resolute Integrity (R, n=8), or BP-DES Nobori (N, n=8) stents were then implanted in each iliac artery. Scanning Electron Microscopy results at 28 days demonstrate that the percentage of uncovered strut area was lowest for SYNERGY (S, n=8) followed by Resolute Integrity (R, n=8), and highest for Nobori (N, n=8) (overall P<0.001). The difference between SYNERGY and Nobori was statistically significant.1
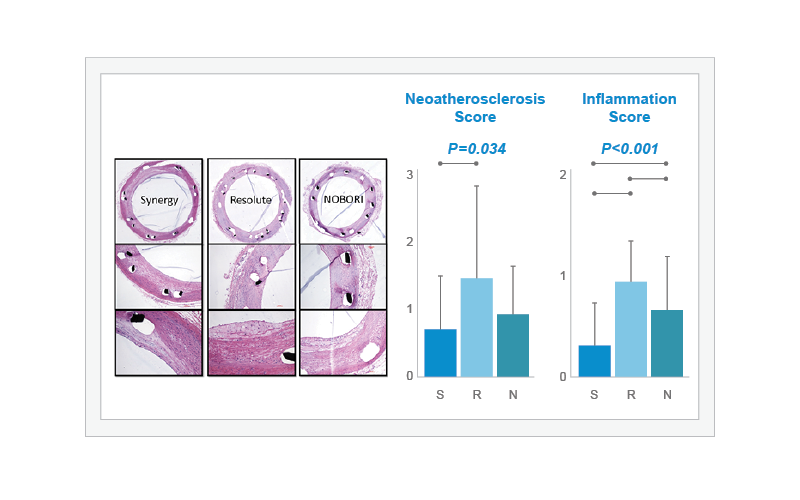
 Neoatherosclerosis and inflammation were compared in SYNERGY (S, n=7), Resolute Integrity (R, n=6), and Nobori (N, n=7) through histological assessment at 90 days. The ‘Neoatherosclerosis Score’ for SYNERGY was lowest across all stents and was statistically lower than Resolute Integrity (overall P=0.034). The histological analysis also demonstrated that the ‘Inflammation Score’ was significantly lower in SYNERGY-treated arteries than in those treated with either Resolute Integrity or Nobori (overall P<0.001).
Together, these data support superior vessel healing with the SYNERGY stent compared to other BP-DES or permanent polymer-DES.
1 Nakazawa G, Torii S, Ijichi T, Nagamatsu H, Ohno Y, Kurata F, Yoshikawa A, Nakano M, Shinozaki N, Yoshimachi F, Ikari Y. Comparison of Vascular Responses Following New-Generation Biodegradable and Durable Polymer-Based Drug-Eluting Stent Implantation in an Atherosclerotic Rabbit Iliac Artery Model. J Am Heart Assoc. 2016; 5:e003803doi:10.1161/JAHA.116.003803.
Neoatherosclerosis and inflammation were compared in SYNERGY (S, n=7), Resolute Integrity (R, n=6), and Nobori (N, n=7) through histological assessment at 90 days. The ‘Neoatherosclerosis Score’ for SYNERGY was lowest across all stents and was statistically lower than Resolute Integrity (overall P=0.034). The histological analysis also demonstrated that the ‘Inflammation Score’ was significantly lower in SYNERGY-treated arteries than in those treated with either Resolute Integrity or Nobori (overall P<0.001).
Together, these data support superior vessel healing with the SYNERGY stent compared to other BP-DES or permanent polymer-DES.
1 Nakazawa G, Torii S, Ijichi T, Nagamatsu H, Ohno Y, Kurata F, Yoshikawa A, Nakano M, Shinozaki N, Yoshimachi F, Ikari Y. Comparison of Vascular Responses Following New-Generation Biodegradable and Durable Polymer-Based Drug-Eluting Stent Implantation in an Atherosclerotic Rabbit Iliac Artery Model. J Am Heart Assoc. 2016; 5:e003803doi:10.1161/JAHA.116.003803.
Angioscopic Evidence of Healing with SYNERGY at 1 to 3 Years
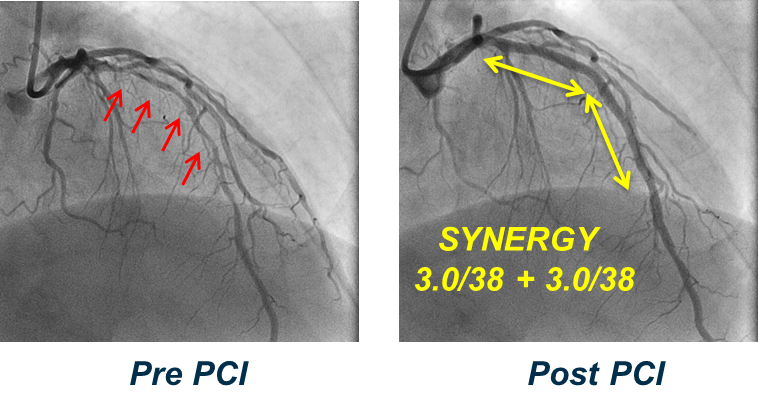
Two SYNERGY stents were implanted in a diffusely diseased left anterior descending artery in a 58 year-old male with angina at rest. Thirteen months following stent implantation, angioscopic images demonstrated covered struts with a thicker neointima (compared to second generation DES), similar to bare metal stents. Thirty-four months following implantation, angioscopic images demonstrated continued complete strut coverage with neither red thrombus nor neoatherosclerosis visible. These angioscopic images provide evidence of continued healing out to three years with the SYNERGY stent.2
2 Ueno, T. Presented at TCT 2016.
Case study not necessarily representative of all cases. Results in other cases may vary.
The SYNERGY Stent Receives FDA Approval for Use in Diabetic Patients
SYNERGY received FDA approval for use in patients with Diabetes Mellitus. The EVOLVE II Diabetes Substudy was a prospective, single-arm, multi-center, non-randomized study that included 466 diabetic patients treated with the SYNERGY Stent (263 patients from the SYNERGY Cohort of the EVOLVE II RCT and 203 patients from the single-arm Diabetes Study). The primary endpoint was Target Lesion Failure (TLF) at 12 months compared to a performance goal based on historical results in diabetic patients. The 12-month TLF rate of 7.5% was well within the performance goal of 14.5% (P<0.0001).3
View Recent SYNERGY Clinical Presentations