Get your latest Longevity report
Capacity + Chemistry + Efficiency

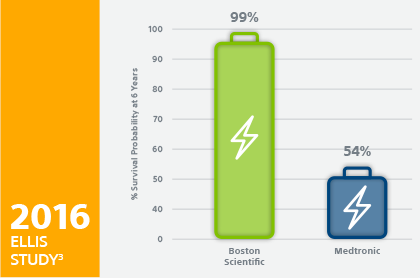
Outlasting the Competition
Nine clinical studies⁴⁻¹² show that devices with EnduraLife Battery Technology are lasting longer than devices from leading competitors.

We're here to help
References:
- Boston Scientific ICDs and CRT-Ds with contemporary battery technology have 1.8 Ah. Medtronic ICDs and CRT-Ds have 1.0 Ah. Amplia MRI CRT-D Surescan DTMB2D4 UK 2016 Manual pg 30. Viva XT CRT-D OUS Manual DTBA2D1 2013 page 28. Evera XT DR ICD OUS Manual DDBB2D4 2014 page 28. DYNAGEN™ EL ICD, DYNAGEN™ MINI ICD, INOGEN™ EL ICD, INOGEN™ MINI ICD, ORIGEN™ EL ICD, ORIGEN™ MINI ICD Physician's Technical Manual. Part Number: 359050-003 EN US 2014-01. DYNAGEN™ CRT-D, DYNAGEN™ X4 CRT-D, INOGEN™ CRT-D, INOGEN™ X4 CRT-D, ORIGEN™ CRT-D, ORIGEN™ X4 CRT-D Physician's Technical Manual. Part Number: 259049-004 EN US 2014-04.
- PHYSICIAN’S TECHNICAL MANUAL DYNAGEN™ EL ICD, DYNAGEN™ MINI ICD, INOGEN™ EL ICD, INOGEN™ MINI ICD, ORIGEN™ EL ICD, ORIGEN™ MINI ICD 2014 page 27-29. PROTECTA™ XT VR D314VRM 2013 page 330. EVERA™ XT VR DVBB1D4 2013 page 24. AnalyST™, AnalyST Accel™, Current™, Current Accel™, Fortify™, Fortify™ ST, Promote™, Promote Accel™, Promote™ Q, Unify™ Devices User’s Manual 2013 page 29. St. Jude Medical™ High-Voltage Devices User’s Manual 2013 page 16.
- Ellis CR, Dickerman DI, Orton JM, Hassan S, Good EG, Okabe T, Andruilli JA, Quan KJ, Greenspon AJ. Ampere Hour as a Predictor of Cardiac Resynchronization Defibrillator Pulse Generator Battery Longevity: A Multicenter Study. PACE 2016 doi: 10.1111/pace.12831 first published online 11-MAR-2016. The five major institutions performing the study include, at Vanderbilt University, Henry Ford Hospital, University of Michigan, Thomas Jefferson University, Cooper Health System, North Ohio Heart Center. Boston Scientific = 322 patients, Medtronic = 794 patients, St. Jude Medical = 186 patients. Five-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- Haarbo J, Hjortshoj S, Johansen J, Jorgensen O, Nielsen J, Petersen H. Device Longevity in Cardiac Resynchronization Therapy Implantable Cardioverter Defibrillators Differs Between Manufacturers: Data from the Danish ICD Registry. Presented at HRS 2014. https://ondemand.hrsonline.org/common/presentation-detail.aspx/15/35/1241/9000. Boston Scientific = 136 patients, Medtronic = 651 patients, St. Jude Medical = 1,587 patients, Bitronik = 369 patients. Time to exchange of the device because of battery depletion or device failure recorded in the Danish ICD Registry was the endpoint. The four-year survival rate for devices in the Danish Registry study was 81.1% for Medtronic and 95.7% for Boston Scientific (P<0.01).
- J. Williams, R. Stevenson. Contemporary cardiac resynchronization implantable cardioverter defibrillator battery longevity in a community hospital heart failure cohort. Presented at HFSA 2014. https://www.onlinejcf.com/article/. S1071-9164(14)00389-3/fulltext. Boston Scientific = 53 patients, Medtronic = 28 patients, St. Jude Medical = 10 patients. Four-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- Ellis CR, Dickerman DI, Orton JM, Hassan S, Good EG, Okabe T, Andruilli JA, Quan KJ, Greenspon AJ. Ampere Hour as a Predictor of Cardiac Resynchronization Defibrillator Pulse Generator Battery Longevity: A Multicenter Study. PACE 2016 doi: 10.1111/pace.12831 first published online 11-MAR-2016. The five major institutions performing the study include, at Vanderbilt University, Henry Ford Hospital, University of Michigan, Thomas Jefferson University, Cooper Health System, North Ohio Heart Center. Boston Scientific = 322 patients, Medtronic = 794 patients, St. Jude Medical = 186 patients. Five-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- Landolina M, Curnis A, Morani G, Vado A, Ammendola E, D’onofrio A, Stabile G, Crosato M, Petracci B, Ceriotti C, Bontempi L, Morosato M, Ballari GP, Gasparini M. Longevity of implant Cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace2015;17:1251-58. doi:10.1093/eurospace/euv109. First published online: May 14, 2015. Medtronic = 532 patients, Boston Scientific = 291 patients, St. Jude Medical = 106 patients, Biotronik = 20 patients, Sorin = 69. Five-year survival rate of latest marketed devices (between 2006 to 2010) calculated using device replacements for battery depletion as indicated by ERI.
- Zanon F, Martignani C, Ammendola E, Menardi E, Narducci ML, De Filippo P, Santamaria M, Campana A, Stabile G, Potenza DR, Pastore G, Iori M, La Rosa C, and Biffi M. Device Longevity in a Contemporary Cohort of ICD/CRT-D Patients Undergoing Device Replacement. Doi:10.1111/jce.12990, First published online 20-APR-2016. Comparison of device longevity by Kaplan-Meier curves of CRT-D systems extracted between March 2013 and May 2015. Medtronic = 195 patients, Boston Scientific = 157 patients, St. Jude = 72, Biotronik = 9.
- Provided by Dr. Ernest Lau on 04/29/15 in support of Lau E, Wilson C, Ashfield K, McNair W, McEneany D, Roberts M, Large Capacity LiMnO2 Batteries Extended CRTD Longevity in Clinical Use Compared to Smaller Capacity LiSVO Batteries Over 6 Years. Presented at HRS 2015. Medtronic = 62 patients, Boston Scientific = 27 patients, St. Jude = 66 patients. Five-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- von Gunten S, Schaer BA, Yap SC, Szili-Torok T, Kühne M, Sticherling C, Osswald S, Theuns DA. Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace. 2015 Nov 25; Epub 2015 Nov 25. Total patients = 3436.
- Alam MB, Munir MB, Rattan R, Adelstein E, Jain S, Saba S. Battery longevity from cardiac resynchronization therapy defibrillators: differences between manufacturers and discrepancies with published product performance reports. Europace 016;doi:10.1093/europace/euw044. First published online: 22-MAR-2016. Kaplan Meier curves depicting survival of CRT devices free from battery depletion by device manufacturer. Battery Longevity in Cardiac Medtronic = 416 patients, Boston Scientific = 173 patients, St. Jude Medical = 57 patients. Previously evaluated these patients at a four-year survival rate calculated using device replacements for battery depletion as indicated by ERI. 2014; Europace (2014) 16,246-51.
- Shabanna Din, Shabanna, Mcgee, Rao, Archana, Wright, Jay D. Longevity of implantable cardioverter defibrillators: The impact of device manufacturer and device type on device longevity were assessed. Europace. 2015 Nov 25; Epub 2015 Nov 25. Total patients = 3436. Cardiostim Abstract 2016. Total patients = 1489.
- https://www.nice.org.uk/guidance/mtg33
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. Material not intended for use in France.
All cited trademarks are the property of their respective owners.
















